Professor Zhu Wei's Team Publishes Paper in Small: "Hardening Tumors" via Biosilicification to Empower T-Cell Cytotoxicity
Recently, the research team led by Professor Zhu Wei from the School of Bioscience and Bioengineering, South China University of Technology (SCUT), published a research paper entitled Stiffening the Soft Tumor: Biosilicification-Enabled Mechanical Immune Checkpoint Blockade Sono-Immunotherapy in the internationally renowned journal Small (IF=12.1). The co-first authors of the paper are Zhang Yuqi (Master's student at SCUT), Chen Yun (Postdoctoral Fellow at Nanyang Technological University, Singapore), and Lin Muyuyang (Master's student at SCUT). The corresponding authors are Professor Zhu Wei, Dr. Ouyang Qing from the Southern Theater Command General Hospital of the Chinese People's Liberation Army, and Dr. Chen Yun.
Paper link: https://onlinelibrary.wiley.com/doi/full/10.1002/smll.202506982
Although immune checkpoint blockade therapy has achieved breakthroughs in tumor treatment, it faces a practical dilemma: only a minority of patients benefit from it, as most tumors can evade immune system attacks through certain mechanisms. Researchers have found that the softness of cancer cells is one of the key factors enabling them to escape immune recognition—by becoming softer, cancer cells make it difficult for T cells to exert effective force and induce cytotoxicity. Therefore, targeting this mechanical immune checkpoint with targeted intervention strategies has emerged as a new direction to improve therapeutic efficacy.
Extrinsic regulation of organisms using functional components to enhance their structure and function is an emerging biological modification strategy. Previously, Professor Zhu Wei's research group has conducted extensive studies around this strategy (Adv. Mater. 2025, 37, 2417050; Adv. Mater. 2024, 2407831; PNAS 2024, 121, e2408273121; PNAS 2024, 121, e2322418121; Angew. Chem. Int. Ed. 2024, 63, e20240611).
Recently, Professor Zhu Wei's team, in collaboration with Dr. Ouyang Qing, proposed a novel synergistic therapeutic strategy: combining in-situ biosilicification with sonodynamic therapy (SDT) to block mechanical immune checkpoints, thereby enhancing the overall efficacy of sonoimmunotherapy.
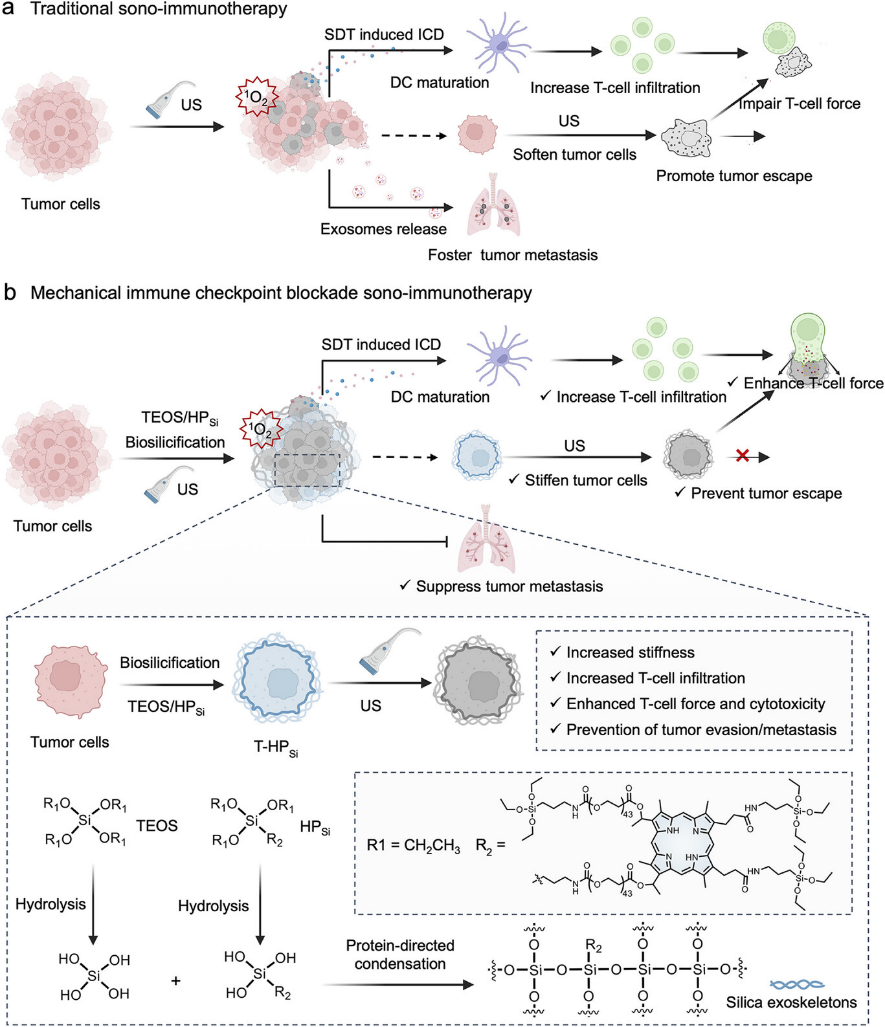
Figure 1. Schematic illustration of in situ biosilicification-assisted mechano-modulation of tumor cells for enhanced MICB sono-immunotherapy. a) The mechanism of traditional sono-immunotherapy contributes to tumor evasion and promotes metastasis. b) The mechanism of the tumor cell biosilicification process and MICB sono-immunotherapy, which enhances the effectiveness of SDT while reducing the side effects typically associated with traditional sono-immunotherapy.
Key highlights of this research include:
Enhancing T-cell attack capability: Regulating tumor cell stiffness to improve T-cell recognition and killing of cancer cells;
Inducing immunogenic cell death: Using a hematoporphyrin-silane conjugate as a sonosensitizer to promote T-cell infiltration into tumor tissues;
Counteracting treatment side effects: Biosilicification effectively alleviates tumor cortex softening caused by SDT and blocks escape pathways;
Inhibiting tumor metastasis: This process suppresses exosome secretion and reduces the risk of distant tumor metastasis.
This method eliminates reliance on expensive and poorly stable artificial antibodies, providing a novel and multifunctional supplementary strategy for existing immunotherapeutic regimens, and demonstrating promising potential for clinical translation.
This work was supported by the Guangdong Basic and Applied Basic Research Foundation, the National Natural Science Foundation of China, Guangdong Provincial Pearl River Talents Program, the Program for Guangdong Introducing Innovative and Entrepreneurial Teams, Natural Science Foundation of Guangdong Province, China, Science and Technology Project of Guangzhou, China, the Fundamental Research Funds for the Central Universities of China.