In the evolving landscape of tumor immunotherapy, monoclonal antibodies (mAbs) have traditionally operated as solitary warriors, targeting single antigens with limited coordination. While effective to a degree, this approach pales in comparison to the potential of multispecific antibodies—the next generation of therapeutic agents capable of waging coordinated 'combinatorial warfare' against cancer. However, the development of these advanced therapeutics has long been hampered by significant technical challenges in their production and application.
Now, a groundbreaking advancement from South China University of Technology (SCUT) is set to change this paradigm. Professor Jun Wang's team at the School of Biomedical Science and Engineering has pioneered an innovative 'Nano-Adaptor' technology that transforms conventional monoclonal antibodies into sophisticated nano-multispecific antibodies. This revolutionary approach not only overcomes longstanding production bottlenecks but also equips these biological agents with enhanced multivalency, multispecificity, and multifunctionality—effectively creating an elite force of cancer-fighting therapeutics.
Overcoming the Limitations of Conventional Approaches
The promise of cancer immunotherapy has been partially realized through monoclonal antibodies targeting immune checkpoints. Yet their clinical impact remains constrained by inherent limitations of single-target recognition. Multispecific antibodies, capable of simultaneously engaging multiple targets on both tumor cells and immune cells, represent a significant leap forward in therapeutic potential. These sophisticated molecules can precisely modulate multiple signaling pathways, offering more comprehensive tumor suppression.
Traditional methods for producing multispecific antibodies—including DNA recombination and protein engineering techniques—have faced formidable obstacles. These approaches typically suffer from low yields, excessive byproduct formation, and complex purification requirements. Moreover, the structural integrity of antibody molecules is often compromised during manufacturing, leading to issues of degradation, aggregation, and denaturation. These challenges have significantly impeded both the development and clinical translation of multispecific antibody therapies.
The Nano-Adaptor Breakthrough
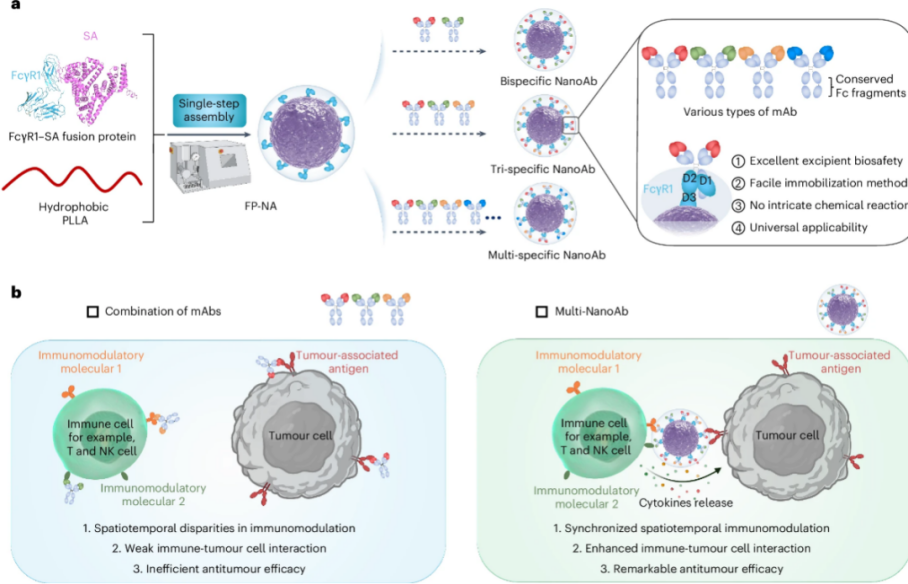
SCUT's research team has developed an elegant solution to these challenges through their innovative Nano-Adaptor platform. By engineering nanoparticles functionalized with multiple monoclonal antibodies, the team has created a system that mimics the natural function of multispecific antibodies while avoiding the pitfalls of conventional production methods.
At the core of this technology lies a universal antibody immobilization platform. The researchers conjugated anti-Fc antibodies—capable of recognizing the conserved Fc region of monoclonal antibodies—onto nanocarrier surfaces. When combined with two or more monoclonal antibody drugs, this system enables the efficient, controllable assembly of nano-multispecific antibodies. The platform addresses three critical challenges that have hindered clinical translation: cumbersome production processes, compromised binding affinity during antibody conjugation, and concerns regarding nanocarrier biosafety.
To extend the military analogy, where conventional monoclonal antibodies operate as isolated units lacking strategic coordination, the Nano-Adaptor technology serves as a sophisticated command center that organizes these individual soldiers into a highly coordinated fighting force. This transformation represents not just an incremental improvement, but a fundamental enhancement in therapeutic capability.
Advancing Toward Clinical Application
Building on this foundation, the research team has further refined their technology through the development of the Fusion Protein Nano-Adaptor (FP-NA) system. This upgraded platform employs genetic engineering to create a recombinant fusion protein combining Fc receptors with serum albumin, which is then assembled with polylactic acid (PLA) polymers through a streamlined one-step process.
The FP-NA platform represents a significant advancement in modular therapeutic development. By leveraging natural receptor-ligand interactions between surface Fc receptors and the Fc regions of monoclonal antibodies, the system eliminates the need for complex chemical conjugation procedures. This innovation enables rapid, flexible development of multivalent, multispecific antibody combinations with enhanced therapeutic potential.
In preclinical studies, FP-NA-assembled nano-multispecific antibodies have demonstrated remarkable efficacy across multiple tumor models, including humanized mouse systems. These engineered therapeutics significantly enhance the ability of immune cells—particularly T cells and macrophages—to recognize and eliminate tumor cells, showcasing their strong clinical potential.
From Laboratory to Patient Care
The SCUT team has successfully completed critical scale-up production trials and technology validation for the Nano-Adaptor platform. This milestone paves the way for clinical translation, with the potential to revolutionize antibody therapeutic development and open new treatment avenues for cancer and other diseases.
Published in the prestigious journal *Nature Biomedical Engineering* under the title 'Engineering multi-specific nano-antibodies for cancer immunotherapy,' this research highlights SCUT's leadership in biomedical innovation. The work, with postdoctoral researcher Yannan Fan as first author and Professors Jun Wang and Song Shen as corresponding authors, exemplifies the university's commitment to fostering original innovation through its distinctive postdoctoral training system and multidisciplinary research environment.
This breakthrough not only represents a significant advance in cancer immunotherapy but also demonstrates how innovative engineering approaches can overcome longstanding biological challenges, bringing us closer to more effective treatments for patients worldwide.





