


Highlights:
1. The intrinsic electrocatalytic performance of the catalyst was evaluated by introducing oxygen defects into the PBSCF film model system.
2. Hydrogen heat treatment and electrochemical reduction were used to introduce oxygen defects with different concentrations, and the existence of oxygen vacancies was proved by XRD and XPS.
3. Electrochemical experiments combined with first-principle calculations revealed the effect of oxygen defects in PBSCF on oxygen catalytic activity: excess oxygen vacancies promoted the adsorption of OH- and reduced the theoretical energy of OH-to form O* on the catalyst surface, which greatly accelerated the OER kinetics. On the other hand, however, oxygen vacancies also increase the energy band gap of PBSCF and reduce the O 2p center, which may hinder the kinetics of OER.
4. Hydrogen heat treatment and electrochemical reduction are applied to practical electrode materials: powders and nanotubes. Different treatment methods can effectively improve the OER performance of PBSCF powder, but the nanotubes after heat treatment agglomerate and the hollow structure of the nanotubes collapses, which reduces the specific surface of PBSCF nanotubes and the OER catalytic performance. Fortunately, the OER performance of electrochemically reduced nanotubes was significantly improved (the overpotential of electrochemically reduced PBSCF nanowires (~ 80 nm) was 313 MV (relative to the reversible hydrogen electrode) and the Tafel slope was 58 MV dec-1 at 10mA cm-2 (current normalized to the geometric region of the electrode).
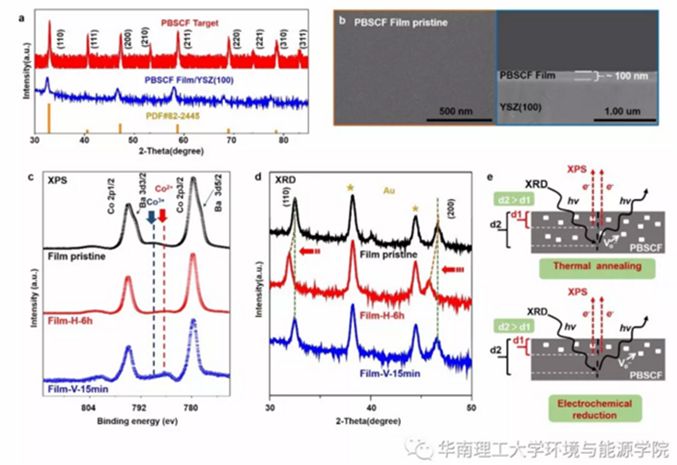
Fig.1 a) XRD patterns of PBSCF target and thin films on YSZ (100) substrates prepared by PLD. b) SEM images of the surface and cross-section of PBSCF thin films. c) Co 2p/Ba 3d XPS spectra and d) XRD patterns of the pristine PBSCF film and the films subjected to 6 h thermal annealing in H2 at 400 °C (Film-H-6h) and 15 min electrochemical reduction with a negative bias of −1.0 V (vs Ag/AgCl) (Film-V-15 min). e) Schematics shows the difference in oxygen vacancy distribution in PBSCF thin film subjected to thermal annealing and electrochemical reduction. d1 and d2 are the probing depth of XPS and XRD.
To avoid the complication arising from the changes in microstructure, PBSCF thin film grown by PLD on inert Yttrium stabilized ZrO2 (YSZ) (001) single crystal substrates were used as the model systems for investigating the effects of oxygen vacancies on the intrinsic OER activity. The thickness of the thin film was estimated to be around 100 nm (Figure 1b). By introducing oxygen vacancies through hydrogen heat treatment and electrochemical reduction, it is obvious that PBSCF with oxygen defect has an obvious CO2+ satellite peak signal in XPS spectrum. In XRD, the lattice of PBSCF after hydrogen heat treatment expanded, while the XRD of PBSCFafter electrochemical reduction changed little. The difference between XPS and XRD results is mainly due to the deeper depth of oxygen vacancies caused by hydrogen heat treatment, while electrochemical reduction treatment is more superficial.
The Tafel slopes and the Rct decreased as thermal annealing time was increased.This indicated that oxygen vacancies generated by thermal annealing and electrochemical reduction cannot be filled back during OER test, and the enhancement in the OER activity due to oxygen vacancies can be sustained after long time operation

Fig.2 a) The polarization curves, b) Tafel plots, and c) Nyquist plots of OER for the PBSCF thin films subjected to thermal annealing (Film-H-xh), respectively. d) Chronopotentiometric curves of the thermally annealed samples (Film-H-xh) at 10 mA cm−2.
The PBSCF films containing oxygen vacancies with higher concentrations of surface oxygen and oxygen cavities are more favorable for the OER reaction. The PBSCF films containing oxygen vacancies have higher surface Sr content, indicating easier generation of Sr-OH. The Gibbs free energy of the tachyon step (*OH-*O) decreases from 0.70 eV to 0.33 eV for PBSCF containing oxygen vacancies with Co as the active site by Gibbs free energy analysis of different steps.

Fig.3 a,b) XPS spectra of a) O 1s, and b) Sr 3d of the pristine PBSCF film, Film-H-6h and Film-V-15 min. c) The computed Gibbs free energy diagram of the four-step 4e− OER mechanism on PBSCF slab with and without one surface vacancy. The adsorption sites investigated include the Co and Fe sites for PBSCF, the Co, Fe, and Vo sites for PBSCF with oxygen vacancy (PBSCF-Vo). d) Atomic structures of PBSCF slabs with adsorbates on the Co site with and without oxygen vacancy presented. The free energy changes of the *OH−*O step with and without induced defect are marked.
The PBSCF containing oxygen vacancies increased the energy band gap of the material from the original 0.75 ± 0.08 eV to 0.94 ± 0.10 eV (containing oxygen defects). The decrease in the O 2p center of PBSCF containing oxygen holes is confirmed by the DOS results, and the above results are detrimental to the improvement of the OER performance.
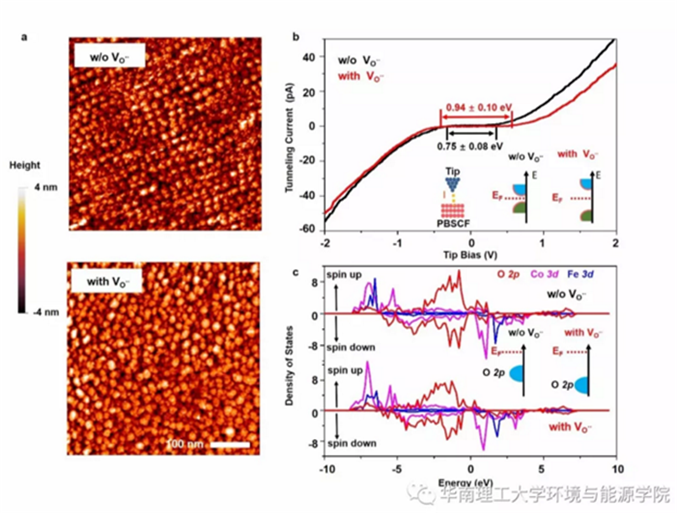
Fig.5. a) STM images (Vtip = 2.5 V, Itunel = 80 pA) and b) tunneling current spectra of the untreated film (w/o VO..) and the one subjected to 400 °C thermal annealing for 2 h in 10−6 mbar H2 environment (with VO..); c) DOS of the O 2p, Fe 3d, and Co 3d states for PBSCF with and without oxygen defect.
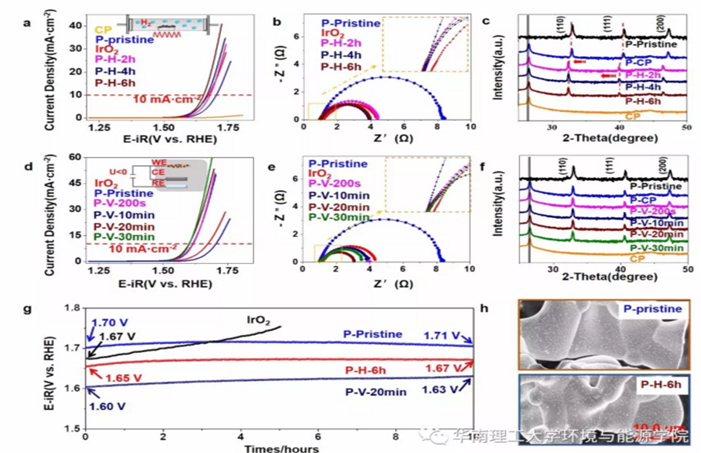
Fig.6 a,d) The polarization curves, b,e) Nyquist plots of OER (measured at V = 1.67 V vs RHE), and c,f) XRD patterns of the pristine PBSCF powders and the ones subjected to thermal annealing and electrochemical reduction. g) Chronopotentiometric curves of the P-pristine, P-H-6h, P-V-20 min and IrO2 catalysts at 10 mA cm−2. h) SEM images of P-pristine and P-H-6h.
The OER properties of the hydrogen heat-treated PBSCF nanotubes (~200 nm) were decreased by agglomeration, the collapse of the hollow structure, and the decrease of the specific surface. The morphology of the nanotubes of PBSCF after electrochemical reduction did not change dramatically. Hence, the hollow structure of the nanotubes was well maintained, and the OER properties of the PBSCF nanotubes after electrochemical reduction were improved. In particular, the OER performance of the electrochemically reduced ~80 nm nanotubes was significantly improved with an overpotential of 313 mV (with respect to the reversible hydrogen electrode) and a Tafel slope of 58 mV dec-1 for the electrochemically reduced nanowires at 10 mA cm-2 (the geometric region normalized to the electrode by the current).
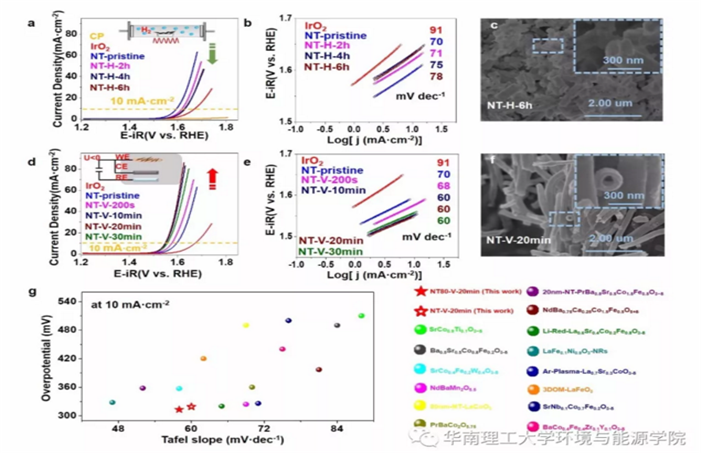
Fig.7 a,d) The polarization curves of OER, b,e) Tafel plots of the PBSCF NT (≈200 nm) and after H2 thermal annealing (H) and electrochemical reduction (V), respectively; c,f) SEM images of the NB-H-6h and NB-V-20 min sample. g) The overpotential at 10 mA cm−2 and Tafel slopes of PBSCF NT subjected to electrochemical reduction (NT-PBSCF-V-20 min and NT80-PBSCF-V-20 min) in this work are compared with recently reported advanced perovskite catalysts.
Details of the essay: https://doi.org/10.1002/adfm.201901783




