1、Synthesis, structure and properties of rare-earth metal complexes

Rare-earth metal complexes have important applications in magnetic and optical materials, and also show great potential in small molecule activation and synthesis of organic compounds and polymers. The synthesis of new rare-earth complexes not only expands the knowledge boundaries of rare-earth chemistry, but also provides the important material basis for the applications of rare-earth. Our group is currently working on the following two areas:
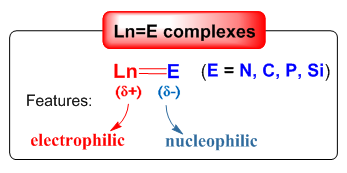
(a) Rare-earth metal-main group element double bond complexes: Complexes containing transition metal-main group element double bonds are not only of great theoretical significance, but also have some important applications. Many transition metal complexes containing metal-main group element double bonds have been synthesized, with the exception of rare-earth metal complexes. The orbital energies of rare-earth ions and alkylidene (imido and phosphinidene) ligands are mismatched, in general the double bonds of rare-earth metal-main elements are extremely unstable. We have achieved some significant progress in the synthesis, characterization and reactivity of the rare-earth metal terminal imido, alkylidene and phosphinidene complexes (see Acc. Chem. Res. 2018, 51, 557; Acc. Chem. Res. 2023, 56, 3343).
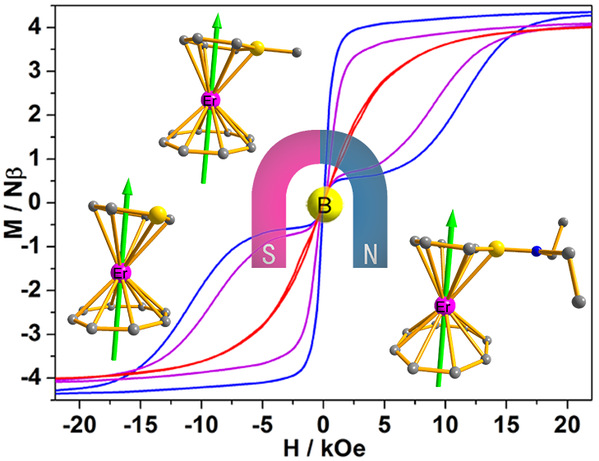
(b) Rare-earth single-molecule magnets: Single-molecule magnets are molecular-sized magnets that exhibit magnetic properties similar to macroscopic magnets on a microscopic level, so they have broad application prospects in ultra-high-density information storage materials, spintronic devices and quantum computing technology. Due to the unique 4f orbital characteristics of rare-earth metal ions, rare-earth metal complexes are the promising direction for the development of high-performance single-molecule magnets. We have established cooperation with Prof. Song Gao's group and have achieved some results in the research of rare-earth single-molecule magnets (see Inorg. Chem. Front. 2016, 3, 828; CCS Chem. 2020, 2, 362; Inorg. Chem. Front. 2023, 10, 485). Based on our advantages in the synthesis of rare-earth metal complexes, we will use the platform of the Spin-X Institute to strengthen the research on the rare-earth single-molecule magnets.
2、Synthesis, structure and properties of silylenes

Silylenes are the silicon analogues of carbenes, which show some similarities with carbenes and also have its own characteristics. According to the difference in their spin state, there are singlet silylenes and triplet silylenes. The substituents on the silicon atom have an important effect on the spin state, stability and reactivity of the silylenes. Based on our own developed metal silyl reagents (Angew. Chem. Int. Ed. 2021, 60, 3189), our group are conducting research in the following two aspects:
(a) Synthesis, characterization and reactivity of metal-substituted silylenes: At present, the synthesized silylenes are mainly based on N, P, O, S substituents, and a small amount based on C and Si substituents. Theoretical calculations show that the silylenes containing the metal substituents have the small ∆ES-T values, and the silylenes ground states containing the strong electropositive metal substituents can even be triplet. We are synthesizing the metal-substituted silylenes, investigating the effects of metal ions on the spin states and the ∆ES-T values, and studying the reactivity of metal-substituted silylenes. Some results have been achieved on the generation and transfer of transient zinc-substituted silylenes (Sci. China: Chem. 2024, 67, 1256).
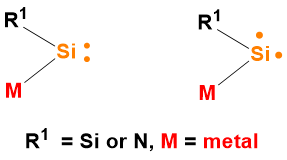
(b) Asymmetric transfer reactions of silylenes: Asymmetric transfer reactions of carbenes have been extensively studied, and a large number of carbon-center chiral compounds have been synthesized by using these reactions. Due to the lack of reaction precursors and the poor stability of reaction intermediates, asymmetric transfer reactions of silylenes remains a big challenge. We are taking this challenge, aiming to synthesizing silicon-center chiral compounds efficiently via silylene asymmetric transfer.
 中文
中文