报告题目1:The power of yocto-liter inner-spaces to affect reactions
报告时间:2023年6月6日(星期二)早上9:00
报告地点:化机3号楼215室
报告题目2:Probing solute-water and solute-anion interactions: Deep-dives into the hydrophobic effect and the Hofmeister effect(s)
报告时间:2023年6月9日(星期五)早上9:00
报告地点:逸夫工程馆四楼大会议室
在线会议地址:
https://teams.microsoft.com/l/meetup-join/19%3aKC6pGctm1yCdq1DNXSYDk1no_MesHBtpDkLa2eLueNk1%40thread.tacv2/1685494257745?context=%7b%22Tid%22%3a%22fe4cdee3-948a-418a-bd27-fb8fcc6fec12%22%2c%22Oid%22%3a%22b8b5e296-d497-46c3-af6c-6b1dd3806c85%22%7d
报 告 人:Bruce Gibb 教授(Tulane University, US)
主 持 人:唐浩教授
化学与化工学院
2023年5月31日
报告1摘要:
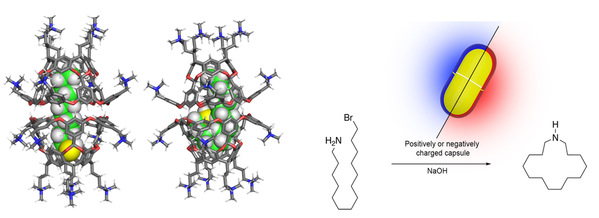
Nature’s catalysts such as enzymes utilize yoctoliter (10–24 L) spaces to facilitate the conversion of their substrates. Creating such spaces in water, de novo, is however no easy task. Approaches can involve the design and folding of foldamers, or they can utilize a brick-by-brick synthetic approach potentially combined with self-assembly. Either way, the state-of-the-art has yet to reach the heady heights of enzyme catalysis.
Our approach to yoctoliter inner-spaces is to take advantage of the self-assembling predisposition of deep-cavity cavitands. Driven by the hydrophobic effect, these form dimeric, supramolecular capsules that engender an inner-space for guest molecule encapsulation. This presentation will discuss the synthesis of the subunits, how they are solvated by water, as well as the types of guests that can be encapsulated in their inner-spaces. Building on this foundational information, the presentation will also detail the ability of these compartments to either selectively prevent reaction and bring about kinetic resolutions, or selectively promote reactions by affecting/controlling the physicochemical properties of encapsulated guests.
References
1)Wang K, Cai X, Yao W, Tang D, Kataria R, Ashbaugh HS, Byers LD, Gibb BC. Electrostatic Control of Macrocyclization Reactions within Nano-spaces. J. Am. Chem. Soc., 2019, 141, 6740–7. DOI: 10.1021/jacs.9b02287.
2)Cai X, Kataria R, Gibb BC. Intrinsic and Extrinsic Control of the pKa of Thiol Guests inside Yoctoliter Containers. J. Am. Chem Soc., 2020, 142(18), 8291-8. DOI: 10.1021/jacs.0c00907.
3)Ashbaugh HS, Gibb BC, Suating P. Cavitand Complexes in Aqueous Solution: Collaborative Experimental and Computational Studies of the Wetting, Assembly, and Function of Nanoscopic Bowls in Water. J. Phys. Chem. B. 2021, 125(13), 3253-68. DOI: 10.1021/acs.jpcb.0c11017.
4)Yao W, Wang K, Ismaiel YA, Wang R, Cai X, Teeler M, Gibb BC. Electrostatic Potential Field Effects on Amine Macrocyclizations within Yoctoliter Spaces: Supramolecular Electron Withdrawing/Donating Groups. J. Phys. Chem. B. 2021, 125(32), 9333-40. Epub 2021/08/07. DOI: 10.1021/acs.jpcb.1c05238.
5)Ismaiel YA, Gibb BC. Water-Soluble Yoctoliter Reaction Flasks. In: Raynal M, Van Leeuwen P, editors. Supramolecular Catalysis - New Directions and Developments: Wiley-VCH; 2021. p. 519-36.
报告2摘要:
A better understanding of how molecules interact in aqueous solutions has ramifications across biochemistry, biotechnology, and biology. However, for multiple reasons — the general weakness of the interactions, the context dependent solvation properties of water, and the approximations used in classic physical models to give just three examples — this area of aqueous supramolecular chemistry is replete with open questions.
We have taken a multi-pronged approach to this problem. On one hand we have synthesize a series of water-soluble hosts possessing well-defined, non-polar pockets. En masse these cavitand hosts have allowed us to probe both the hydrophobic effect, and ion-solute interactions that are in part responsible for the Hofmeister effect(s).1 On the other hand, we also study more complex proteinaceous systems such as the protein Ubiquitin,2 or intrinsically disordered and well-folded peptides. Despite their greater complexity, these solutes tell us much about the strength and quality of their interactions with water and co-solute ions.
This presentation will cover both these topics, summing up our contribution to moving the field of aqueous supramolecular chemistry forward, and highlighting what we still don’t know about how solutes, ions and water interact.
References
1. (a) Jordan, J. H.; Gibb, C. L. D.; Wishard, A.; Pham, T.; Gibb, B. C., Ion-Hydrocarbon and/or Ion-Ion Interactions: Direct and Reverse Hofmeister Effects in a Synthetic Host, J. Am. Chem. Soc., 2018, 140 (11), 4092-4099; (b) Carnagie, R., Gibb, C. L. D. and Gibb, B. C., Angew. Chem. Int. Ed. 2014, 53, 11498-11500; (c) Barnett, J. W.; Sullivan, M. R.; Long, J. A.; Tang, D.; Nguyen, T.; Ben-Amotz, D.; Gibb, B. C.; Ashbaugh, H. S., Spontaneous drying of non-polar deep-cavity cavitand pockets in aqueous solution. Nat. Chem. 2020, 12 (7), 589-594. d) Suating, P, Ernst, N. E., Alagbe, B. D., Skinner, H. A., Mague, J. T., Ashbaugh, H. S., Gibb, B. C., On the Nature of Guest Complexation in Water: Triggered Wetting-Water-Mediated Binding, J Phys Chem B 2022, 126 (16), 3150-3160.
2. Yao, W.; Wang, K.; Wu, A.; Reed, W. F.; Gibb, B.C., Anion binding to ubiquitin and its relevance to the Hofmeister effects, Chemical Science 2021, 12 (1), 320-330.
报告人简介:
Bruce C. Gibb是图兰大学的化学教授。他以在水相超分子化学领域的工作而闻名,特别强调自组装导致的区域化,并对疏水效应和霍夫迈斯特效应(例如在不同盐存在下的蛋白质溶解度)做出了概念层面上的贡献。其主要研究领域包括有机超分子主体合成、主客体识别及分子组装、超分子纳米反应器和药物分子传输等方向的研究。
Gibb教授是《超分子化学》的共同主编,并自2009年以来一直是《Nature Chemistry》的特邀专栏评论员。自2015年起,他成为国际大环和超分子化学研讨会(ISMSC)国际咨询委员会的成员,并于2018年当选为皇家化学学会会士。Gibb教授主持了包括美国NSF和NIH在内的多项基金,其研究成果发表在如自然-化学 (Nature Chemistry), 美国国家科学院院刊 (PNAS), 美国化学会志(J. Am. Chem. Soc.), 德国应用化学 (Angew. Chem. Int. Ed.)以及化学会综述(Chem. Soc. Rev)等高水平杂志上,其重要发现曾被Nature杂志进行过专文介绍,并受邀撰写多部专著及章节。