报告题目:Oxygen Vacancy in Energy Conversion and Storage Devices – a Defect or a Beauty?(能量转换和储存装置中的氧空位—缺陷还是美丽?)
报 告 人:张山青 教授
报告时间:2018年11月6日(星期二)下午3:30-4:30
报告地点:逸夫工程馆105会议室
欢迎广大师生踊跃参加!
化学与化工学院
2018年11月6日
报告人简介:
Prof. Shanqing Zhang obtained his PhD degree in electrochemistry in 2001 at Griffith University, Australia. Since then, he has worked as a research fellow during 2001-2006, senior research fellow during 2007-2009 and associate professor at Centre for Clean Environment and Energy and Griffith School of Environment, Griffith University during 2010-2015. As a core inventor, Dr. Zhang has developed a series of patented and commercialized technologies for environmental monitoring based on functional nanomaterials. He was awarded Australia Research Council Future Fellow for 2009-2013. Currently, Prof. Zhang is mainly engaged in the research on synthesis of functional nanomaterials and modification of environmentally friendly natural polymers for energy conversion and storage devices, as well as the development of intelligent online and/or onsite sensors for environmental monitoring. He has published ca. 150 reputable papers and delivered numerous Keynotes and invited lectures.
报告摘要:
Oxygen Vacancy in Energy Conversion and Storage Devices – a Defect or a Beauty?
Shanqing Zhang
Centre for Clean Environment and Energy, School of Environment and Science, Gold Coast Campus, Griffith University, QLD 4222, Australia
Email: s.zhang@griffith.edu.au
Abstract: Oxygen vacancy (OV) can be considered as a defect when the number of oxygen atoms expected in a compound is less (or missing) than what it should be in its perfect crystal lattice, it is known as In this talk, OV in titania and lithium titanate nanomaterials achieved via numerous ways (hydrogenation, cold quenching and chemical reduction) is characterized and applied for photocatalysis and lithium ion battery applications. The preliminary results suggest that the OV could bring about significant enhancement in energy conversion efficiency and energy storage capability and stability.
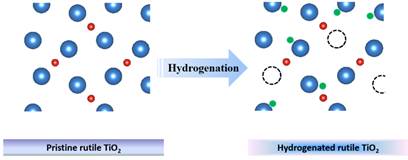
Figure1, Hydrogenation, a classical way to obtain oxygen vacancy.
References
1. Chen, H., Ling, M., Hencz, L., Ling, H. Y., Li, G., Lin, Z., & Zhang, S. (2018). Chemical Reviews. 2018 118 (18), 8936-8982.
2. D. Adekoya, X Gu, M. Rudge, W. Wen, C Lai, M Hankel, & S. Zhang, Advanced Functional Materials, 2018, DOI: 10.1002/adfm.201803972.
3. Qiu J., Li S., Gray E., Liu H., Gu Q., Sun C., Lai C., Zhao H., Zhang S., J. Phys. Chem. C., 2014, 118, 8824−8830