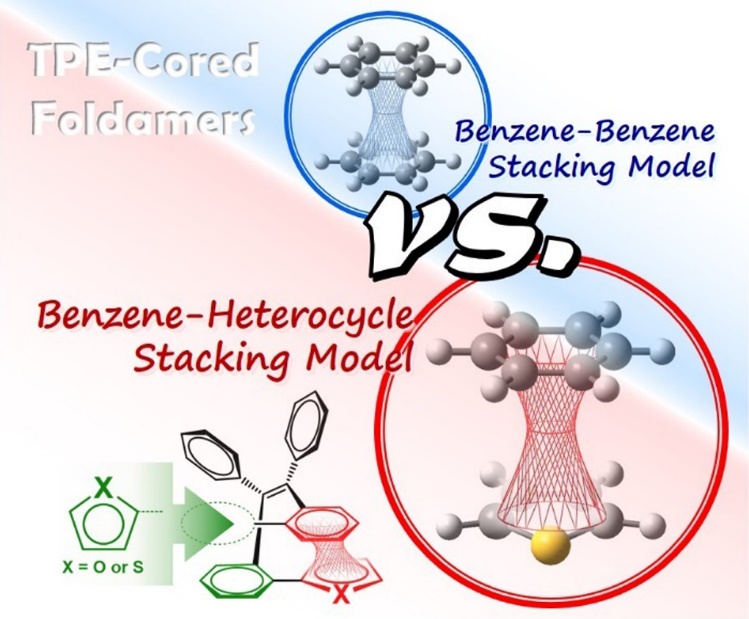
Conjugation, as an essential chemical term used to describe electron delocalization, can be roughly grouped into two categories, through-bond conjugation (TBC) and through-space conjugation (TSC). A hybrid conjugation system integrating both TBC and TSC is rarely studied and utilized, for lack of a well-established model and difficulty of structure modification and property tuning, despite its theoretical significance and potential applications. Herein, various foldamers with a tetraphenylethene (TPE) core are employed as hybrid conjugation models to investigate structure–property correlation by introducing heterocycles of furan/thiophene into the π-stacking TSC component. For comparison, two kinds of TPE-cored foldamers with different stacking models, a benzene–heterocycle stacking model and a benzene–benzene stacking model, are designed. Combining experimental measurements and theoretical calculations, the impact of benzene–heterocycle interaction on the hybrid conjugation natures and photophysical properties has been studied systematically. The results reveal that the benzene–heterocycle stacking model can fabricate a hybrid conjugation nature with an improved TSC component to make a more dominant contribution to the electronic transition natures than the benzene–benzene stackingmodel, leading to the distinguishing photophysical behavior. This work provides valuable guidance for the design of new functional materials with hybrid conjugation systems.
Ph. D. candidate Zeyan Zhuang in our group is the first author of this article, and the related paper is available online at CCS Chemistry (DOI: 10.31635/ccschem.021.202000677).
