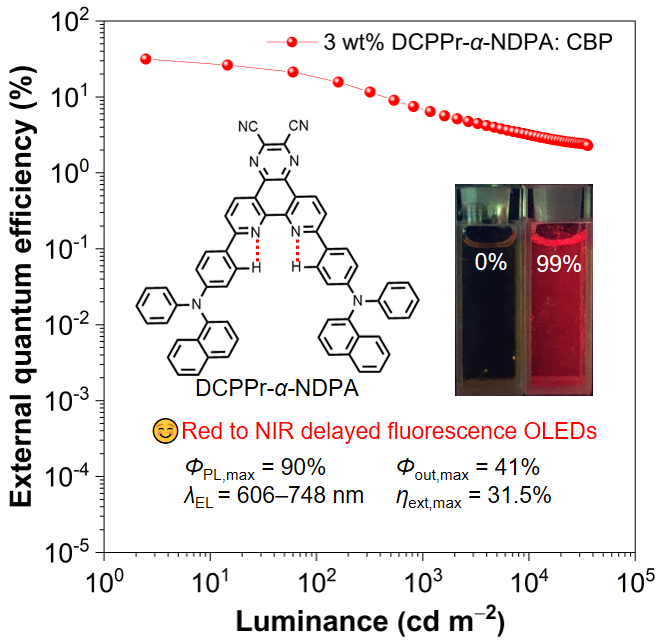
Purely organic molecules with thermally activated delayed fluorescence (TADF) hold an advantage of high exciton utilization in organic light-emitting diodes (OLEDs). However, robust TADF materials with emission peaks over 600 nm are still insufficient due to strong non-radiative decay according to energy-gap law. Herein, tailor-made red TADF molecules comprised of an electron-withdrawing pyrazino[2,3-f][1,10]phenanthroline-2,3-dicarbonitrile core and various electron-donating triarylamines are developed. These molecules can form intramolecular hydrogen-bonding, which is conducive to improving emission efficiency and promoting horizontal orientation by increasing molecular rigidity and planarity. They show near infrared (NIR) emissions (692–710 nm) in neat films and red delayed fluorescence (606–630 nm) with highphotoluminescence quantum yields(73–90%) in doped films, and prefer horizontal orientation with large horizontal dipole ratios in films, rendering high optical out-coupling factors (0.39–0.41). Their non-doped OLEDs exhibit NIR lights (716–748 nm) with maximum external quantum efficiencies (ηext,maxs) of 1.0–1.9%. And their doped OLEDs radiate red lights (606–648 nm) and achieve outstanding ηext,maxs of up to 31.5%, which is the highest value for TADF materials emitting over 600 nm ever reported. These red TADF materials should have great potentials in displays and lighting devices.
Ph. D. candidate Zheyi Cai in our group is the first author of this article, and the related paper is available online at Angewandte Chemie International Edition (DOI: 10.1002/anie.202111172).
