Surface Functionality-Regulated and Entropy-Driven Thermodynamics of the Formation of Coordination Nanocages
Shuqian He†, Mingxin Zhang†, Binghui Xue†, Yuyan Lai†, Mu Li†, Panchao Yin*
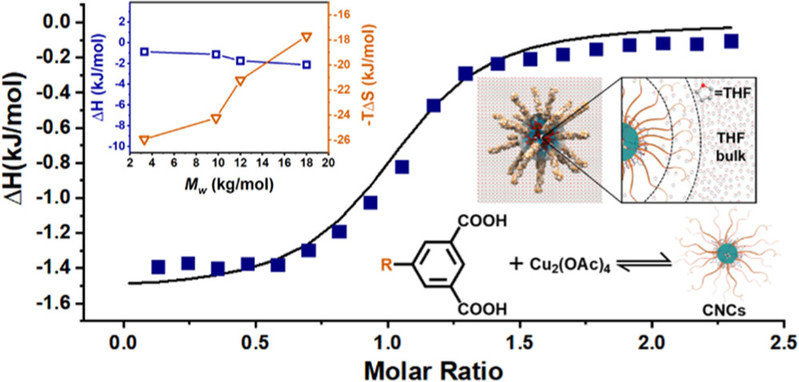
Coordination Nanocages (CNCs) are under intense research in nanoscience and supramolecular chemistry for their enriched surface functionalities and micro-porosity; however, the understanding of their formation mechanism is still poor due to the difficulty in probing their solution structures. Herein, the CNC formation process from the coordination complexation of macromolecular isophthalic acid (IPA) ligand and Cu2+ is studied via isothermal titration calorimetry and its entropy-driven feature is revealed to be originated from the collapse of solvation layers of the assembly units. The CNC formation is thermodynamically less favored with smaller binding constants when the sizes of macromolecular IPA ligands are larger, originated from the space crowding of macromolecules of the ligands on CNC surfaces and the resulting entropy loss of polymer chain conformations. Meanwhile, the chemical equilibrium of CNC formation can be tuned upon the altering of Cu2+/IPA ratio and the yield of CNC, suggested from size exclusion chromatography studies, decreases when excessive Cu2+ are applied, providing guidelines for CNC design and synthesis.
配位纳米笼(CNCs)由于其丰富的表面功能和微孔隙度而受到纳米科学和超分子化学的广泛研究;但由于其溶液结构难以探测,对其形成机理的认识尚不充分。本文采用等温滴定量热法研究了大分子邻苯二甲酸(IPA)配体与Cu2+形成CNC配位配合物的过程,揭示了其熵驱动特征源于组装单元溶剂化层的坍塌。当大分子IPA配体尺寸较大时,由于大分子配体在配位纳米笼表面的空间拥挤和聚合物链构象的熵损失,导致其热力学配位过程的热力学结合常数较小,不利于配位纳米笼的形成。同时,Cu2+/IPA比值的改变可以调节配位纳米笼形成的化学平衡,尺寸排除色谱研究表明,当使用过量Cu2+时,配位纳米笼的产率降低,为配位纳米笼的设计和合成提供了指导。
文章链接:https://pubs.acs.org/doi/10.1021/acs.jpcb.1c06690

