Hydrogen bonding directed co-assembly of polyoxometalates and polymers to core-shell nanoparticles
Jing Zhou,ab Jie Hu,a Mu Li,a Hui Li,b Weiyu Wang,a Yuzi Liu,c Randall E. Winans,d Tao Li,*ed Tianbo Liu*b and Panchao Yin![]() *a
*a
* Corresponding authors
a South China Advanced Institute for Soft Matter Science and Technology & State Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510640, China
b Department of Polymer Science, The University of Akron, Akron, USA
c Center for Nanoscale Materials, Advanced Photon Source, Argonne National Laboratory, Argonne, USA
d X-ray Science Division, Advanced Photon Source, Argonne National Laboratory, Argonne, USA
e Department of Chemistry and Biochemistry, Northern Illinois University, DeKalb, USA
Mater. Chem. Front., 2018, 2, 2070-2075
DOI: 10.1039/C8QM00291F
Publication Date (Web): September 13, 2018
Copyright © The Royal Society of Chemistry and the Chinese Chemical Society 2018
E-mail: yinpc@scut.edu.cn; tliu@uakron.edu; taoli@aps.anl.gov; tli4@niu.edu
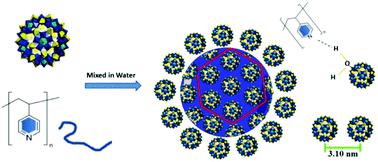
A general strategy has been developed here to co-assemble polyoxometalates (POMs) and polymers into core-shell hybrid nanoparticles via hydrogen bonding interaction. Due to the hydrogen bonds between the pyridine groups of poly(4-vinyl pyridine) (P4VP) and the hydrogen bonding donor groups on the POM surface, P4VP is stabilized by the POMs and dispersed as discrete hybrid core-shell nanoparticles in aqueous solution. For these thermodynamically stable nanoparticles, the P4VP cores are covered with hexagonal close-packed POMs. The size of the core-shell particles is controlled by the electrostatic repulsive interaction among the POMs. The introduction of extra salts screens the repulsive force among the POMs, thus increasing the size of the core-shell structures.

