Wenqing Zhang lab has published an article in Leukemia showed that Asxl1 C-terminal mutation perturbs neutrophil differentiation in zebrafish.
Myeloid malignancies are characterized by the proliferation and defective differentiation of myeloid progenitors[1, 2]. The main types of these malignancies are myelodysplastic syndrome (MDS), myeloproliferative neoplasms (MPNs), and acute myeloid leukemia (AML). Patients with multiple myeloid malignancies frequently harbor somatic mutations in addition of sex combs-like 1 (ASXL1) [3]. In patients, ASXL1 mutations are usually heterozygous frameshift or nonsense mutations leading to C-terminal truncation[3] and loss of the ASXM2 domain and a PHD finger. Truncated ASXL1 mutations independently predict poor outcomes and shorter overall survival for patients with CMML.[4, 5] However, it is still largely unknown how ASXL1 participates in normal hematopoiesis and leukemogenesis.
In January 22, 2021, a research team led by professor Wenqing Zhang from South China University of Technology and Kuangyu Yen from Southern Medical University published their recent work in Leukemia titled “Asxl1 C-terminal mutation perturbs neutrophil differentiation in zebrafish”. Their work indicated that impaired neutrophil differentiation may be the fundamental for the progression of myeloid malignancies.
Fig. 1: C-terminally truncated with asxl1 mutations specifically blocked neutrophil differentiation.
To elucidate the function of Asxl1 in hematopoiesis and the leukemogenic mechanisms of Asxl1-truncated mutants, they established an endogenous truncated mutant in zebrafish that is comparable to human disease. At the embryonic stage, neutrophil differentiation was explicitly blocked. WISH and qRT-PCR at 3 dpf embryos showed significantly reduced lyz (a mature neutrophil marker) and mpx (a intermediate neutrophilic progenitor marker) transcripts. Furthermore, FACS-sorted neutrophils from asxl1+/+ and asxl1-/- embryos were May-Grünwald-Giemsa stained to observe neutrophils morphology. Compared with wild type, the asxl1 mutant exhibited a lower proportion of mature neutrophils, whose signature is banded and segmented nuclei. At 6 months, mutants initially exhibited a myelodysplastic syndrome-like phenotype with neutrophilic dysplasia. this finding indicated that asxl1 mutant neutrophils remained in an immature stage. The decrease and immaturity of neutrophils is typical of MDS with neutrophilic dysplasia.
At 1 year, about 13% of mutants further acquired the phenotype of monocytosis, which mimics chronic myelomonocytic leukemia, or increased progenitors, which mimics acute myeloid leukemia. These features are comparable to myeloid malignancy progression in humans. their model demonstrated that neutrophilic dysplasia caused by asxl1 mutation is a foundation for the progression of myeloid malignancies
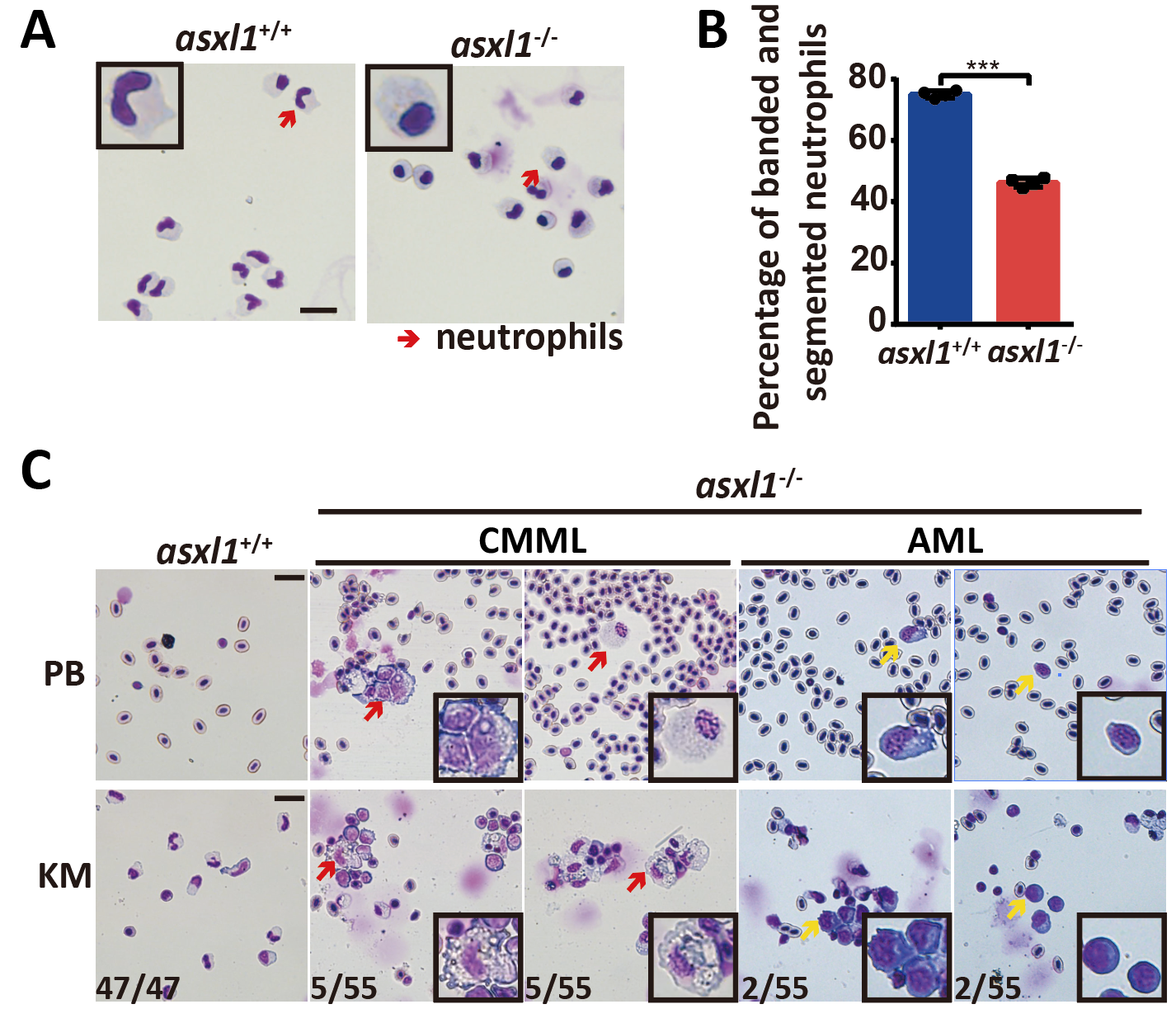
Fig. 2: Zebrafish asxl1 mutant has impaired neutrophil maturation.
To understand the basis for impaired neutrophil differentiation in zebrafish asxl1 mutants, we performed RNA-seq. Similar to the observed phenotypes, expression of all neutrophil markers was downregulated. Expression of myeloid progenitor and macrophage markers was not impaired. Gene ontology analysis showed that wounding response-related genes were significantly enriched in asxl1 mutants, possibly because impaired neutrophils influenced the response to wounding. They also found that genes for an inflammatory cytokine and a matrix metalloproteinase were upregulated in asxl1 mutants, which may be from induction by the deficiency of neutrophil granzymes. RNA-seq data also showed that the expression of bmi1a and cbx4, which encode members of PRC1, was downregulated in asxl1 mutants. bmi1a is a reported myeloid leukemia-associated gene[6] and is associated with the proliferative activity of granulocyte/macrophage progenitor (GMP)[7]. This study through inhibitor treatment and rescue assays indicated that asxl1-induced neutrophilic dysplasia was associated with reduced expression of bmi1a.
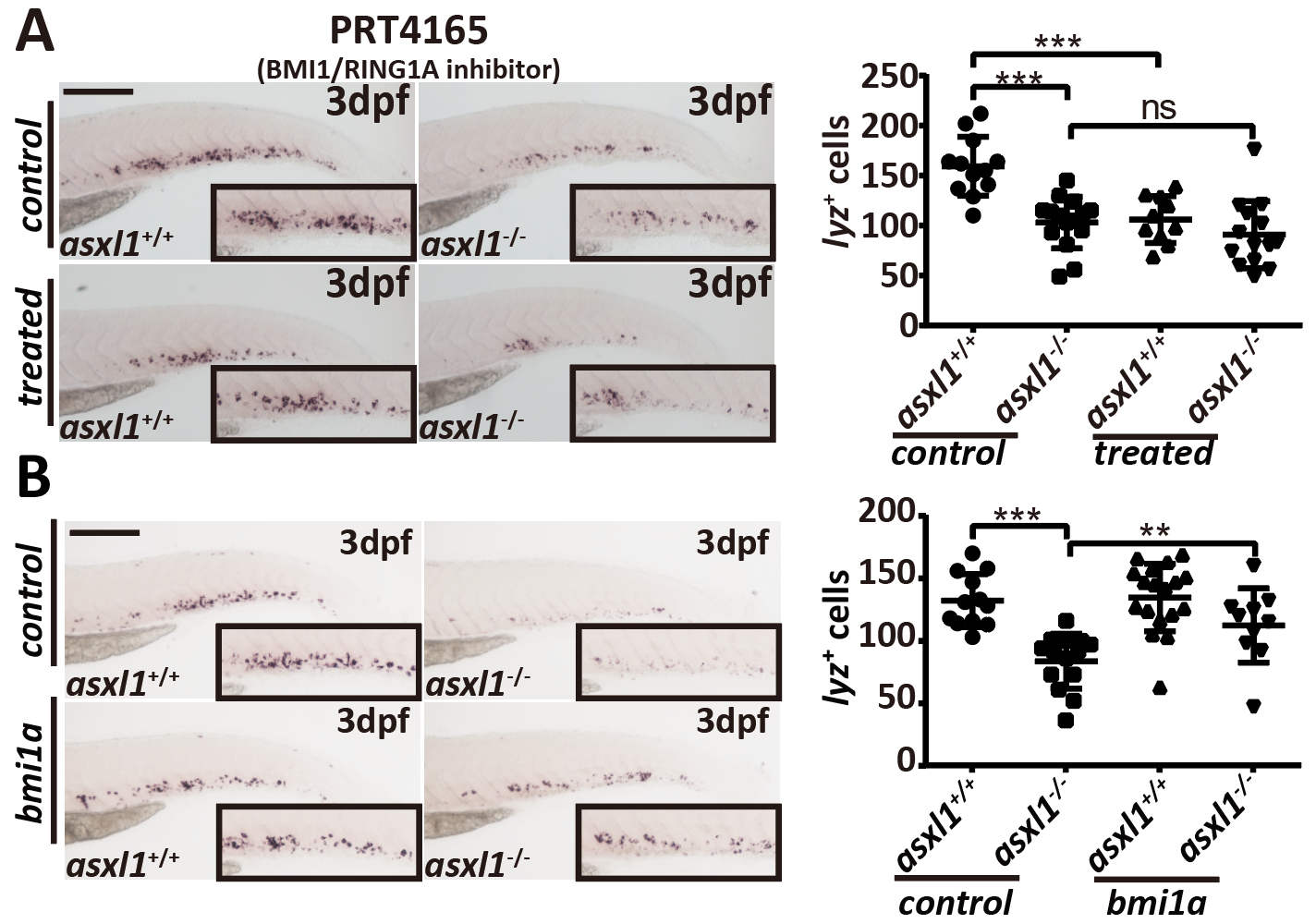
Fig. 3: Inhibition of PRC1 components is associated with disruption of neutrophils development.
Taken together, results from this study show that endogenous truncated asxl1 mutations result in impaired neutrophil differentiation. Their model revealed that neutrophilic dysplasia caused by asxl1 mutations is a foundation for the progression of myeloid malignancies, and bmi1a dysregulation is associated with neutrophil dysplasia.
Xiao Fang, a PostDoc of the South China University of Technology, is the first author of this paper. Prof. Wenqing Zhang from South China University of Technology and Prof. Kuangyu Yen from Southern Medical University are the corresponding authors. The authors also include Song’en Xu, Yiyue Zhang, Jin Xu, Zhibin Huang, Wei Liu and Shunqing Wang.
[1]KORN C, MéNDEZ-FERRER S. Myeloid malignancies and the microenvironment [J]. Blood, 2017, 129: 811-22.
[2]MURATI A, BRECQUEVILLE M, DEVILLIER R, et al. Myeloid malignancies: mutations, models and management [J]. BMC Cancer, 2012, 12(1): 304.
[3]GELSI-BOYER V, BRECQUEVILLE M, DEVILLIER R, et al. Mutations in ASXL1 are associated with poor prognosis across the spectrum of malignant myeloid diseases [J]. J Hematol Oncol, 2012, 5: 12.
[4]GELSI-BOYER V, TROUPLIN V, ROQUAIN J, et al. ASXL1 mutation is associated with poor prognosis and acute transformation in chronic myelomonocytic leukaemia [J]. Br J Haematol, 2010, 151(4): 365-75.
[5]ITZYKSON R, KOSMIDER O, RENNEVILLE A, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia [J]. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 2013, 31(19): 2428-36.
[6]SAHASRABUDDHE A A. BMI1: A Biomarker of Hematologic Malignancies [J]. Biomarkers in cancer, 2016, 8: 65-75.
[7]YUAN J, TAKEUCHI M, NEGISHI M, et al. Bmi1 is essential for leukemic reprogramming of myeloid progenitor cells [J]. Leukemia, 2011, 25(8): 1335-43.

