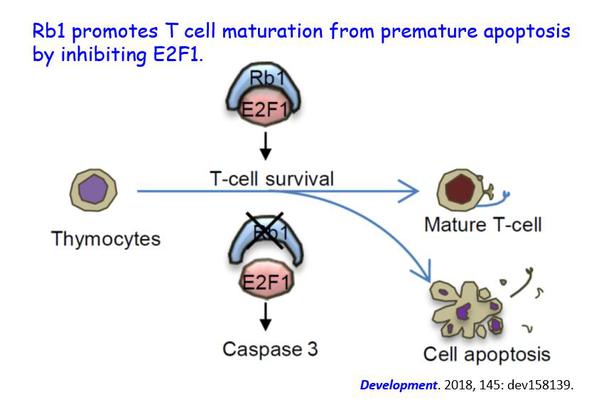
T lymphocytes are key cellular components of immune system and play essential roles in cell-mediated immunity. Recently, Professor Yiyue Zhang’s lab and Professor Wenqing Zhang’s lab discovered that Rb1 is crucial for protecting immature T cells from apoptosis during early T lymphocyte maturation by inhibiting E2F1 in zebrafish. This study provided in vivo evidence of Rb1 function in T lymphocyte differentiation and providing new molecular and cellular basis for the regulation of early T lymphocyte differentiation.
This work entitled “Retinoblastoma 1 protects T cell maturation from premature apoptosis by inhibiting E2F1” was reported in Development (http://dev.biologists.org/content/145/1/dev158139, 2018, 145(1): dev158139). Professor Yiyue Zhang and Professor Wenqing Zhang from Division of Cell, Developmental and Integrative Biology, School of Medicine are co- corresponding authors and Associate Professor Wei Liu is the co-first author. This work was supported by National Natural Science Foundation of China, Ministry of Science and Technology Project 863, and the Team Program of Guangdong Natural Science Foundation.
T cell development occurs in the thymus where 95% of immature thymocytes are eliminated via apoptosis. Previous study has reported that the RB1 interacting protein E2F1 can regulate the development of mature T cell by interfering with thymocyte apoptosis. However, whether Rb1 regulate thymocyte development in vivo is still need to be further investigated. In this study, the team used zebrafish model to investigate the role of Rb1 in T cell development. The results showed that Rb1-deficient fish exhibit a significant reduction of T cells during early development and it is attributed to the accelerated apoptosis of immature T cell in a Caspase-dependent manner. Further studies found that E2F1 overexpression could mimic the reduced T lymphocytes phenotype of Rb1 mutants, and E2F1 knockdown could rescue the phenotype in Rb1-deficient mutants. Collectively, all data indicated that Rb1-E2F1-Caspase axis is crucial for protecting immature T cells from apoptosis during early T lymphocyte maturation. Furthermore, given the fact that somatic deletion of rb1 is the most frequent chromosomal abnormality in CLL, elucidating the mechanism behind Rb1 in immature T cell apoptosis regulation provides an intriguing link between tumor suppression and lymphatic system development.