Topic: Mechanistic Insight in Palladium Catalyzed Stereoselective Allylic Alkylation and Metal-Free Cyclization of Alkynes with Nitriles
Speaker: Dr. Langui Xie, University of Oxford
Time: 9:00, December 26, 2017
Venue: Room 205, Building 14, Wushan Campus
Abstract:
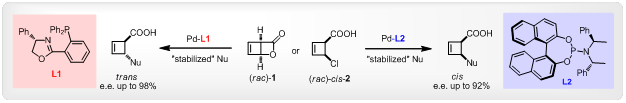
Story I: Allyl palladium(II) complexes represent key intermediates in catalytic allylic substitution (or Tsuji-Trost) reactions. We have recently reported unusually strong ligand effects in the diastereodivergent asymmertric synthesis of disubstituted cyclobutenes through palladium-catalyzed allylic alkylation.[1] With the regard to the mechanistic understanding of this transformation, we will herein present our findings on the unique structure and reactivity of cyclobuten-3-yl-palladium complexes as well as their unprecedented behaviors in solution.[2]
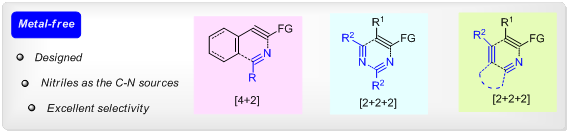
Story II: Nitrogen-containing heteroaromatic cores are ubiquitous building blocks in organic chemistry. Herein, we present a family of metal-free intermolecular formal cycloaddition reactions that enable highly selective and orthogonal access to isoquinolines and pyrimidines at will, as well as rapid assembly of substituted pyridine structures. These methodologies benefit from the strategic use of readily available nitriles as the C-N sources. [3]
References:
[1] M. Luparia, M. T. Oliveira, D. Audisio, F. Frebault, R. Goddard, N. Maulide, Angew. Chem. Int. Ed. 2011, 50, 12631-12635; b) D. Audisio, M. Luparia, M. T. Oliveira, D. Klütt, N. Maulide, Angew. Chem. Int. Ed. 2012, 51, 7314-7317.
[2] a) L.-G. Xie, V. Bagutski, D. Audisio, L. Wolf, V. Schmidts, K. Hofmann, C. Wirtz, W. Thiel, C. M. Thiele, N. Maulide, Chem. Sci., 2015, 6, 5734-5739; b) D. Audisio, G. Gopakumar, L.-G. Xie, L. G. Alves, C.Wirtz, A. M. Martins, W. Thiel, C. Farès, N. Maulide, Angew. Chem. Int. Ed. 2013, 52, 6313-6316.
[3] a) L.-G. Xie, S. Niyomchon, A. J. Mota, L. Gonzalez, N. Maulide*, Nat. Commun., 2016, 7, 10914. b) L-G Xie, S. Shaaban, X. Chen, N. Maulide*. Angew. Chem. Int. Ed., 2016, 55, 12864-12867.





