(Lecture, May. 29) Notice on the academic report of Professor Zhuang Lin of Wuhan University
Title:Mechanism of H2 oxidation reaction under multi-dimensional electrochemical cracking condition
Time: May 29, 10:00-12:00 a.m
Venue: Conference Room 105, Shaw Engineering Building
[Biography]

Zhuang Lin is a research professor and Dean of the School of Chemistry and Molecular Sciences, Wuhan University. In 1993, he graduated from the Department of Chemistry of Wuhan University. In 1998, he received his doctorate degree from Wuhan University. 2004-2005 Visiting Scholar, Department of Chemistry, Cornell University. He was President of ECS China, Vice President of the Physical Electrochemistry Branch of ISE, and deputy editor of Journal of Physical Chemistry and ACS Sustainable Chemistry & Engineering. He is currently the executive deputy Director of the Electrochemistry Committee of the Chinese Chemical Society. He has hosted the National Outstanding Youth Science Fund project, and was selected as a member of the National High-level Talent Special Support Program and the Chinese Chemical Society. Presided over the national key research and development plan projects, NSFC major projects. He has been engaged in hydrogen electrochemistry research for a long time, focusing on the scientific innovation of "zero carbon" and "negative carbon" technologies such as fuel cells, water electrolysis and CO2 reduction, and his research on transformative hydrogen electrochemistry technologies based on alkaline membranes is pioneering and leading.
[Abstract]
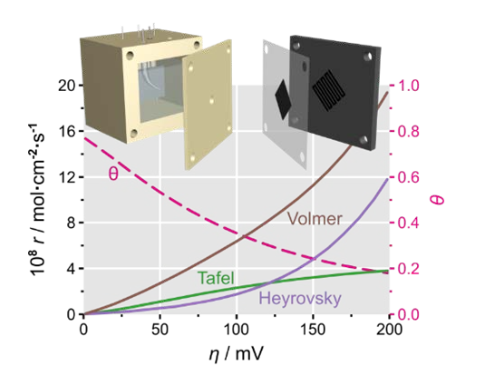
Under the current "two-carbon" background, H2 oxidation reaction (HOR) has attracted much attention as an important way of hydrogen utilization. The mechanism of HOR consists of three elementary reactions: Tafel, Heyrovsky and Volmer. Without any assumptions or approximation, at least five parameters are required to describe the HOR mechanism completely: the reaction rate of the three elementary steps (𝑟T,𝑟H,𝑟V), the coverage of Had of adsorbed hydrogen (𝜃) and the charge transfer coefficient (𝛽). These five parameters cannot be accurately and reliably obtained by fitting the voltammetry curves obtained by electrochemical measurements, so previous studies had to make assumptions and simplify, and were limited to the thermodynamic equilibrium potential, unable to discuss the mechanism of non-equilibrium conditions.
In this study, an electrochemical method to completely crack the HOR working mechanism is reported. First, to break the limitation of hydrogen solubility, a gas diffusion electrode (GDE) is used to ensure that electrochemical measurements over a wide potential range are not affected by mass transfer. Secondly, multi-dimensional electrochemical data are obtained by using both direct current (I-V) and electrochemical AC impedance (EIS) techniques, especially the independent currents (𝑗) and charge transfer resistance (𝑅ct) under polarization conditions, which make it possible to rigor mathematically model the 5-dimensional parameter space. Through 𝑗 and 𝑅𝑅 ct altogether fitting, equilibrium potential can be obtained under the dynamic parameter set (𝑟 T0, 𝑟 H0, 𝑟 where V0, 𝜃 0, 𝛽), and then calculate the HOR under arbitrary polarization reaction rate of each primitive steps and Had coverage changes. The results show that the Volmer reaction is always the fastest in the whole study potential range (0 ~ 200 mV). Since the previous HOR mechanism studies could not obtain a comprehensive dynamic image like this, they assumed that the Volmer reaction was a rate-controlled step, which was obviously wrong. In addition, HOR reaction mechanism changes with polarization conditions. In the small polarization region, Heyrovsky is relatively slow, and HOR can be simplified as Tafel-Volmer mechanism. However, in the large polarization region, the reaction rate of Heyrovsky will exceed that of Tafel, becoming the main hydrogen dissociation step. All these indicate the importance of the study of the operando mechanism. The discussion of the mechanism under the thermodynamic equilibrium condition may not be applicable to the non-equilibrium state under the operating condition of the device.
Announced by School of Chemistry and Chemical Engineering
