The human body is an integrated complex system of many dissimilar components. For instance, the bonding between tendon and cartilage to bone tissues is the structural basis for body movement. From a biomimetic viewpoint, combining materials with drastically different chemical compositions and mechanical characteristics might provide the hybrid systems with superior properties and functions. Such metal–hydrogel hybrid implants might potentially facilitate the success of implant metal devices and bio integration of interfaces at the soft and hard tissues. Moreover, it has been recently shown that the hybridization of hydrogels with metals and other solid substrates may produce new synergistic materials that possess unprecedented levels of structural and functional features, which can be used for hydrogel painting, sensors, and soft robots, among others. For these applications, one critical issue is to form tough and robust bonding between hydrogels and other solid substrates.
Traditional methods include electrochemical and photochemical reactions as well as layer-by-layer depositions, which result in complex adhesion mechanisms but only moderate interfacial adhesion energy. The realization of tough bonding between hydrogels and diverse solid substrates has not been achieved until 2016, Zhao and co-workers extended this silane-based chemical anchoring strategy to various nonporous solid surfaces and achieved tough bonding between them with adhesion energy up to >1000 Jm−2. Furthermore, Suo and co-workers initiated a novel concept of “topological adhesion,” and demonstrated bonding of hydrogels to metal surfaces via stitching polymer chains at the interfaces. However, introduction of the such adhesions still required multiple processes with relatively harsh conditions, and subsequent on-demand debonding of the involved components is still underexplored.
Recently, Prof. Kan Yue’s groupof our college collaborating with Prof. Y. Shrike Zhang from Harvard Medical School rationally reported a new a simple and universal strategy to achieve both tough bonding and on-demand debonding of hydrogels to diverse metal substrates.More interestingly, after the reductive debonding, the resulting metal surface with free thiol groups can be easily rebonded with a second hydrogel without any further surface modification (Figure 1).
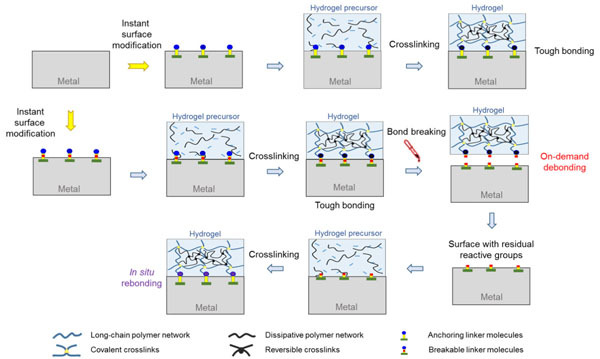
Figure 1. Design strategy for tough bonding, on-demand debonding and facile rebonding of hydrogels to diverse metal surfaces.
They designed a linker molecule (Linker-1), which has a carboxylic acid group to bind with metal surfaces, and a methacrylic group to crosslink with hydrogels, thus bridging the interface between them.Impressively, the measured bonding energy between hydrogels and Linker-1-treated metal surfaces reached as high as 1200 Jm−2, which was comparable to the strong bonding found between natural tendon or cartilage and bones (~ 800 Jm−2). Besides, swelling the bonded hydrogels in neutral (pH = 7), weakly acidic (pH = 5), and basic (pH = 9) buffer solutions for 12 h did not signifcantly lower the bonding energies (Figure 2).
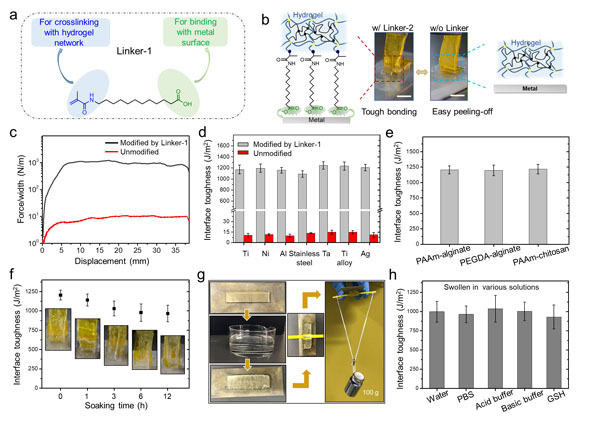
Figure 2. (a) Molecular structure of the Linker-1 as the bridge between hydrogels and metal substrates. (b) Photographs of the peeling tests of tough hydrogels bonded to Ti substrates (left) with and (right) without treatment by Linker-1. Scale bars: 5 mm. (c) Representative force-displacement curves of bonded hydrogel-metal systems with and without treatment by Linker-1. (d) Measured interfacial toughness values of hydrogels bonded to diverse metals via Linker-1. (e) Measured interfacial toughness values for different tough hydrogel systems chemically anchored to Ti substrates. (f) Measured interfacial toughness at different soaking time in DI water. (g) Image showing the capability of a swollen hydrogel attached to a Ti substrate to resist a weight of 100 g. (h) Measured interfacial toughness values of hydrogels bonded to Ti substrates after swelling in different conditions.
Based on these results, it was reasoned that by eliminating the chemical anchorage via an external stimulus, on-demand debonding between hydrogels and metal surfaces could be realized. To achieve this under a milder condition, they modified the chemical structure of Linker-1 by introducing a breakable disulfde bond to prepare the Linker-2 molecule.To debond, the hybrid systems were treated with aglutathione (GSH) solution. Upon breaking the disulfde bond in the linker molecules that bridge the interfaces of hydrogels and metalsthe tough bonding can be released.More interestingly, the residue part of the Linker-2 molecule bonded at the metal surfaces stillcontained a free thiol group, the newly applied hydrogel network was again chemically anchored to the metal surfacevia the thiol-ene addition mechanism (Figure 3).
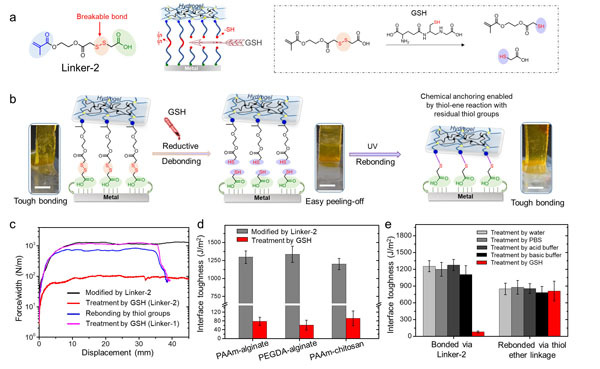
Figure 3. (a) Molecular structure of Linker-2 (left) and the reductive cleavage of the disulfide chemical linkage by GSH (right). (b) Schematic illustration of the experimental processes to achieve tough bonding, on-demand debonding, and subsequent in situ rebonding using Linker-2 as the interfacial bridge. Scale bars: 5 mm. (c) Representative force-displacement curves obtained from the 90-degree peeling tests of bonded hydrogel-Ti systems via Linker-2 (black like), after GSH treatment, and after re-bonding. Result from a control group of bonded hydrogel-metal system via Linker-1 indicated that GSH treatment did not significantly reduce its bonding energy. (d) Measured interfacial toughness value for different tough hydrogel systems chemically anchored to Ti substrates with and without treatment by GSH. (e) Measured interfacial toughness values of hydrogels bonded to Ti via Linker-2 and rebonded after swelling in different conditions.
This research can provide a higher level of flexibility and functionality towards rational design of “smart” materials and devices based on hydrogels and metals for applications in dynamic and complex situations, such as the bonding of metal implants with engineered tissues based on hydrogels. This work has been published on Advanced materials. The Author list: Weichang Li, Xiaobo Liu, Zhishuang Deng, Yutong Chen, Qianqian Yu, Wen Tang, Tao Lin Sun, Yu Shrike Zhang,* and Kan Yue*.
The link of this article is: https://onlinelibrary.wiley.com/doi/10.1002/adma.201904732
