Self-assembly of molecular building blocks provides a versatile platform for engineering desired supramolecular nanostructures. A variety of molecular building blocks with distinct geometries such as the sphere, rod, disc and so on have been exploited. Among them, rod-like building blocks have attracted considerable interests because of their unique photophysical and electrochemical properties. However, dominated by their preferred parallel arrangement, the self-assembly of rod-like molecules generally leads to the formation of nematic or layered smectic structures. In order to construct other supramolecular structures with the curved interface by rod-like molecules and further investigate their macroscopic properties as a function of supramolecular structures, a new molecular design concept based on rod-like components is highly demanded.
Recently, Prof. Stephen Z. D. Cheng research group proposed a new molecular design concept to engineer diverse supramolecular structures of rod-like molecules via rational design of molecular topology and precise manipulation of non-covalent interactions between rods. The designed molecular topology and non-covalent interactions facilitate the unparallel arrangements of rod-like components, leading to diverse self-assembled nanostructures including the Frank-Kasper (F-K) A15 phase and dodecagonal quasicrystal (DQC) (Figure 1.).
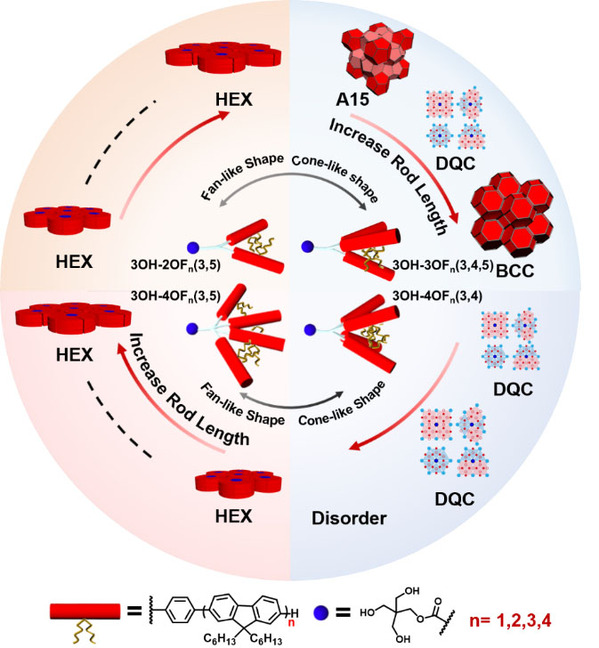
Figure 1. Chemical Structures and Self-assemblies of 3OH-2OFn(3,5), 3OH-3OFn(3,4,5), 3OH-4OFn(3,5) and 3OH-4OFn(3,4).
The Cheng laboratory first precisely synthesized four categories of dendritic oligo-fluorene (OF) rods capped by a tri(hydroxyl) group, referred to as 3OH-XOFn(P). The tri(hydroxyl) group will provide the driving force for the self-assembly. Due to the largely incommensurate cross-section area between the tri(hydroxyl) group and multi-OF rods, the curved interface is preferred. Moreover, two side chains are attached to each OF rod unit, and large steric hindrance between side chains results in weaker or negligible π-π interaction between OF rods. The synergistic effects of the above factors are anticipated to partially break the preferred parallel orientation of OF rods and shape a fan-like or cone-like geometry during self-assemblies.
The researchers then systematically investigated the self-assembly of this set of molecules in bulk. For 3OH-2OFn(3,5) (n=1-4), all samples of these molecules exhibit hexagonal cylindrical (HEX) phases, which could be attributed to the same AB2 dendritic architecture. Specifically, this architecture results in the fan-like conformation that preferentially assembles into disc-like motifs and packs into HEX phases. When the number of OF rods increases to there, the 3OH-3OFn(3,4,5) molecules hold conical conformation and exhibit a phase sequence (F-K A15 → DQC → body-centered cubic (BCC) phases) with increasing rods length (Figure 2). Notably, 3OH-4OFn(3,5) and 3OH-4OFn(3,4) are pairs of constitutional isomers with the same number of n, but they self-assemble to form completely different supramolecular structures. Regardless of the length of the OF rods, only HEX phases are observed in 3OH-4OFn(3,5); while 3OH-4OFn(3,4) can self-assemble to form DQC phases for n=1 and 2. To further reveal the molecular design principle of these molecules, the researchers elucidate the origin of different molecular geometry by proposing a thermodynamic model, in which major contributions of Gibbs free energy to the system are taken into account; besides that, the phase sequence of 3OH-3OFn(3,4,5) has also been well understood.This study provides a model system to engineer diverse supramolecular structures constructing by rod-like molecules and sheds new light into the mechanisms of the formation of unconventional spherical packing structures in soft matter.
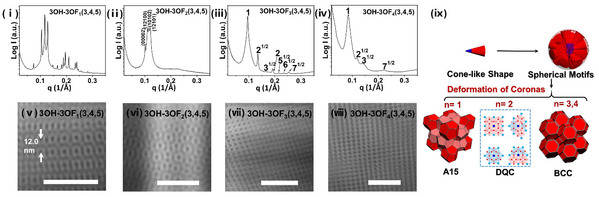
Figure 2. Supramolecular structures self-assembled from 3OH-3OFn(3,4,5). Scale bars represent 50 nm.
This work has been published on Angewandte Chemie International Edition.
Author list: Ruimeng Zhang, Xueyan Feng, Rui Zhang,Wenpeng Shan, Zebin Su, Jialin Mao, Chrys Wesdemiotis, Jiahao Huang, Xiao-Yun Yan, Tong Liu, Tao Li,Mingjun Huang, Zhiwei Lin*, An-Chang Shi*, Stephen Z. D. Cheng*
Angew. Chem. Int. Ed.2019, 58 (34), 11879-11885.
