Hematopoietic stem/progenitor cells (HSPCs), including hematopoietic stem cells and several lineage-biased hematopoietic progenitor cells, provide all blood cell types in adult organisms. Among them, hematopoietic stem cells have the ability of self-renewal and multi-lineage differentiation, and can respond rapidly under acute conditions [1], while a variety of hematopoietic progenitor cells can maintain the supply of blood cells in steady-state hematopoiesis [2]. Therefore, HSPCs are the core of the blood system. Once their homeostasis is disrupted, it will lead to severe blood diseases and even death. Research on HSPCs can provide valuable insights into related theoretical research and clinical treatments. To study the development of the hematopoietic system, researchers often apply zebrafish as the experimental model. Zebrafish has the advantages of the short growth cycle, high fecundity, in vitro fertilization, embryo transparency, and easy access to gene editing, making it an ideal model for developmental biology. In addition, the hematopoietic system of zebrafish is also highly similar to that of humans [3]. This conserveness is also the basis for the application of zebrafish in hematopoietic research. On March 16, 2021, Yiyue Zhang's and Wenqing Zhang’s research groups, both from South China University of Technology, cooperated to publish a research paper titled Slc20a1b is essential for hematopoietic stem/progenitor cell expansion in zebrafish on SCIENCE CHINA Life Sciences (Figure 1). This study revealed that a point mutation of the zebrafish slc20a1b gene would result in severe defects in the expansion of HSPCs, resulting in dramatic impairment of hematopoietic development.

Figure 1. The title page of the published paper.
In 2012, the two research groups cooperated in the forward genetic screening of zebrafish through the ENU-induced random gene mutation, and obtained a series of zebrafish mutant lines [4]. In this study, based on the smu07 mutant obtained by ENU mutagenesis, the authors identified that the mutant had defects in erythroid, myeloid, and lymphoid hematopoiesis, suggesting that its upstream HSPCs might already defect. Further investigations revealed that the mutant HSPCs could be generated normally but failed to expand, thus making the HSPCs of mutants significantly fewer than that of wild-type larvae (Figure 2).
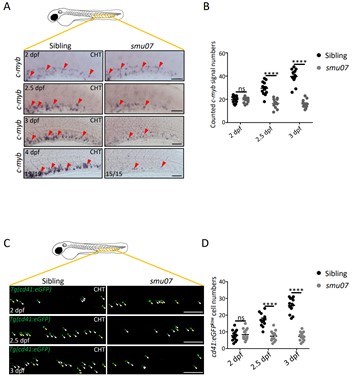
Figure 2. The expansion defects of smu07 HSPCs
The authors performed gene mapping to locate the mutagenic gene and found it to be slc20a1b, which involved a single DNA base mutation resulting in a single amino acid mutation (D48E). By CRISPR-Cas9 technology, the authors generated a slc20a1b mutant zebrafish line, which showed an identical phenotype as the smu07 mutant, verifying that the mutagenic gene is indeed slc20a1b.
Since slc20a1b is widely expressed, the authors further explored whether the mutations in HSPCs contributed to their defects, or other cells in their environment played a dominant role. Through forward and reverse transplantation experiments, the authors found that the mutant HSPCs could not expand in the normal environment, while wild-type HSPCs could expand in the mutant environment. This result demonstrated that the autonomous deficiency of the slc20a1b gene in HSPCs led to their defects.
Finally, the authors explored the mechanism underlying the mutant HSPC defects and found that the cell death of HSPCs upon slc20a1b mutation was unchanged, but the defects in their proliferation abilities prevented the HSPCs from expanding. The cell cycles of mutant HSPCs were blocked at the G2/M phase and thus failed to proliferate.
In conclusion, the HSPCs of the slc20a1b mutants have an autonomous defect in cell cycle progression, leading to decreased proliferation (Figure 3). Thus, the mutants suffer severe insufficiencies of the HSPCs, and the subsequent hematopoietic lineages are all dramatically defected.
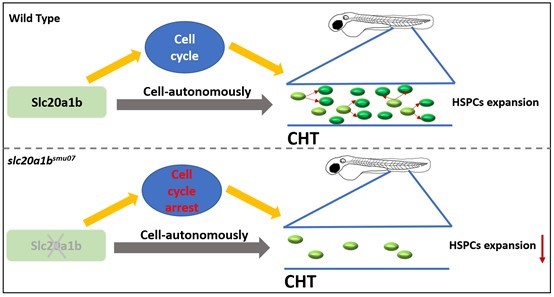
Figure 3. The working model of this research.
This study is the first to report the important role of the slc20a1b gene in HSPCs, revealing new regulatory mechanisms in the cell cycle of HSPCs.
Jiakui Chen, a doctoral student from Southern Medical University, and Dr. Gaofei Li from South China University of Technology are the co-first authors of the paper. Professor Yiyue Zhang and Professor Wenqing Zhang from South China University of Technology are the co-corresponding authors of the paper. Other authors of the paper include Dr. Junwei Lian, Dr. Zhibin Huang, Prof. Jianchao Li from South China University of Technology, Dr. Ning Ma from Southern Medical University, and Prof. Zilong Wen from Hong Kong University of Science and Technology.
References:
1. S.H. Orkin, L.I. Zon, Hematopoiesis: anevolving paradigm for stem cell biology., Cell. 132 (2008) 631–644.
2.K. Busch, K. Klapproth, M. Barile, M.Flossdorf, T. Holland-Letz, S.M. Schlenner, M. Reth, T. Hofer, H.-R. Rodewald,Fundamental properties of unperturbed haematopoiesis from stem cells in vivo.,Nature. 518 (2015) 542–546.
3. B. Bajoghli, N. Aghaallaei, I. Hess, I.Rode, N. Netuschil, B.-H. Tay, B. Venkatesh, J.-K. Yu, S.L. Kaltenbach, N.D.Holland, D. Diekhoff, C. Happe, M. Schorpp, T. Boehm, Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates., Cell. 138(2009) 186–197.
4. K. Wang, Z. Huang, L. Zhao, W. Liu, X.Chen, P. Meng, Q. Lin, Y. Chi, M. Xu, N. Ma, Y. Zhang, W. Zhang, Large-scale forward genetic screening analysis of development of hematopoiesis in zebrafish., J. Genet. Genomics. 39 (2012) 473–480.

